
Yaroslav I. Molkov, Ph.D.
Associate Professor
Georgia State University
Department of Mathematics and Statistics
30 Pryor St SW, 750 COE, Atlanta, GA 30303
Office: 722 COE
Email: ymolkov@gsu.edu
Phone: (404) 413-6422
Skype: yaroslav.molkov

|
Yaroslav I. Molkov, Ph.D.Associate Professor
Georgia State University |
Research and Interests
My current research activity includes elaboration and qualitative analysis of large-scale computational models of mammalian respiratory central pattern generator located in the brainstem and its metabolic state-dependent interactions with other structures. This task involves multivariate time-series analysis of nerve and unit activities, data assimilation approaches, numerical modeling and simulation, asymptotic methods of dynamical systems reduction, qualitative analysis of dynamical systems etc. I have solid background in physics, mathematics and computational neuroscience, strong programming skills and a necessary knowledge of the existing physiological data on the respiratory system to successfully carry out the proposed studies, including processing and analysis of experimental data, computer modeling of the respiratory neurons, neural networks and large-scale neural systems, and analysis of the models using dynamical system theory methods. As a postdoctoral fellow in Prof. Rybak group at Drexel University College of Medicine, I have intensively studied neurophysiology of respiration and significantly contributed to the development of several contemporary computational models of the brainstem respiratory network. These models represent a basis for their further extension for studying respiratory-sympathetic and -parasympathetic coupling as mechanisms probably involved in hypertension, such disorders as Chayne-Stokes and periodical breathing, Rett syndrome and other diseases. During the development of those models I closely collaborated with Dr. Jonathan Rubin at the University of Pittsburg, Dr. Jeffrey Smith at NINDS NIH, Dr. Julian Paton at the University of Bristol, UK, Dr. Thomas Dick at CWRU. Our joint works have been recently published in the Journals of Neurophysiology, Computational Neuroscience and Respiratory Physiology & Neurobiology. In addition, I developed a novel approach to parallel computing for the simulation of large, multi-population neural models and, using this approach, I implemented our large-scale respiratory models in the NIH heterogeneous multiprocessor cluster (BioWulf) using OpenMPI environment.
Respiration in mammals is a primal homeostatic process, regulating levels of oxygen (O2) and carbon dioxide (CO2) in blood and tissues and is crucial for life. Rhythmic respiratory movements must occur continuously throughout life and originate from neural activity generated by specially organized circuits in the brain stem constituting the respiratory central pattern generator (CPG). The respiratory CPG generates rhythmic patterns of motor activity that produce coordinated movements of the respiratory pump (diaphragm, thorax, and abdomen), controlling lung inflation and deflation, and upper airway muscles, controlling airflow. These coordinated rhythmic movements drive the exchange and transport of O2 and CO2 that maintain physiological homeostasis of the brain and body. Uncovering complex multilevel and multiscale mechanisms operating in the respiratory system, leading to mechanistic understanding of breathing, including breathing in different disease states requires an approach that relies on the development and explicit implementation of multiscale computational models of particular organs and physiological functions. The specific aims of this multi-institutional project are: (1) develop a predictive, multiscale computational model of neural control of breathing that links multiple physiological mechanisms and processes involved in the vital function of breathing but operating at different scales of functional and structural organization, (2) validate this model in a series of complementary experimental investigations and (3) use the model as a computational framework for formulating predictions about possible sources and mechanisms of respiratory pattern alteration associated with heart failure. The project brings together a multidisciplinary team of scientists with long standing collaboration and complementary expertise in respiration physiology, neuroscience and translational medical studies (Thomas E. Dick, Case Western Reserve University; Julian F.R. Paton, University of Bristol, UK; Robert F. Rogers, Drexel University; Jeffrey C. Smith, NINDS, NIH), bioengineering (Alona Ben-Tal, Massey University, NZ) and mathematics, dynamical system analysis and computational neuroscience and neural control (provided by our lab). The end result of our proposed cross-disciplinary modeling and experimental studies will be the development and implementation of a new, fully operational, multiscale model of the integrated neurophysiological control system for breathing based on the current state of physiological knowledge. This project is currently being funded by NINDS NIH (2010-2015 Grant# R01 NS069220 PI - Ilya Rybak, Drexel U College of Medicine).
My role in this project is to incorporate a core respiratory CPG model and periphery providing chemo- and baro- afferent inputs and its interactions with sympathetic and parasympathetic nervous systems as well as to orchestrate the experimental studies aimed to verify the model predictions. This integrative work is based on several specific studies described below providing the models of afferent inputs from chemoreceptors, pulmonary stretch receptors, baroreceptors and efferent outputs to sympathetic and para-sympathetic systems respectively.
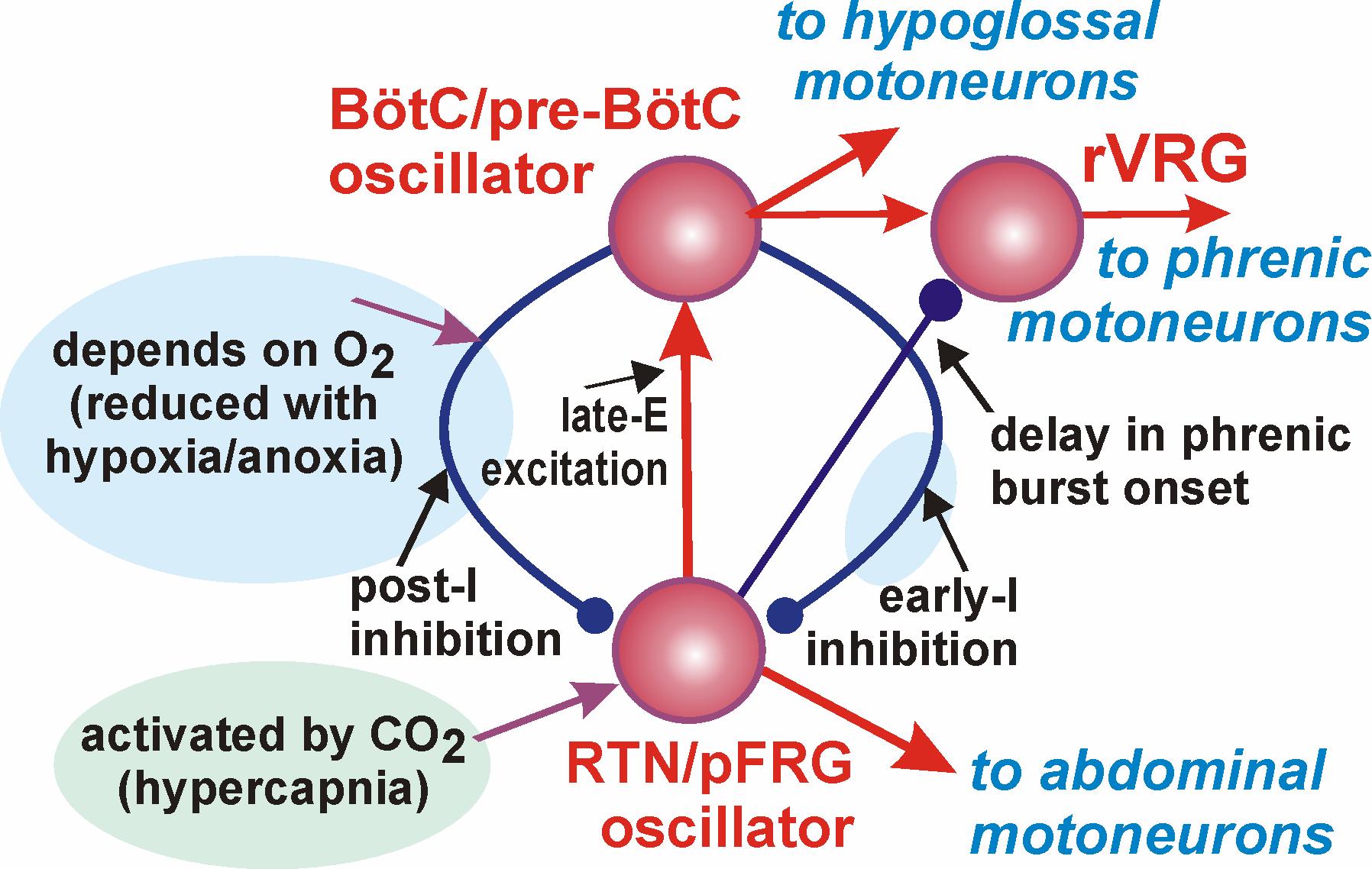
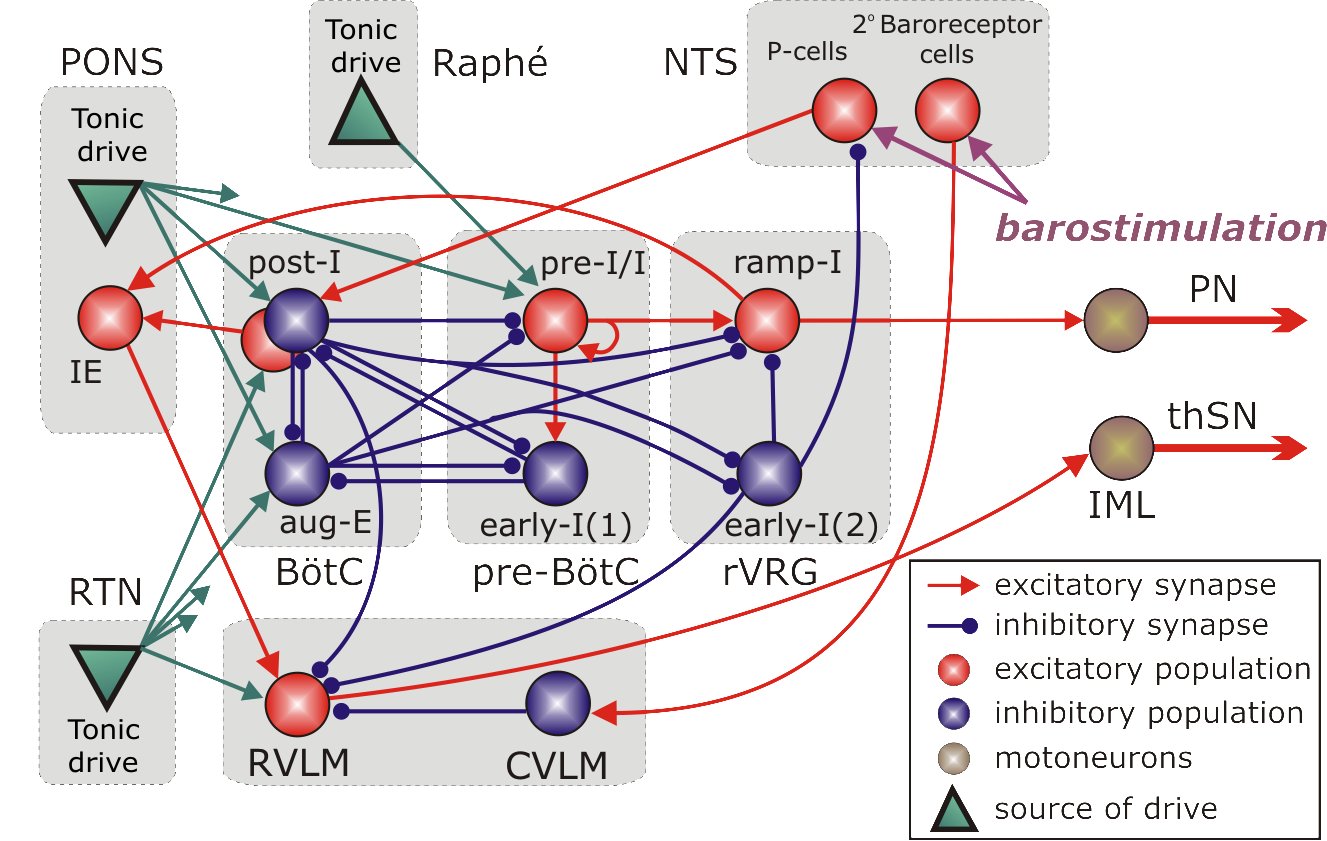
Dysfunctions of respiratory neural control are associated with multiple breathing disorders, including sleep apnea, sudden infant death syndrome, Rett syndrome, and congenital central hypoventilation syndrome. Despite a long history of studies, the neural mechanisms responsible for generation of the respiratory rhythm and pattern and their adaptive re-organization with changing metabolic or physiological conditions are not well understood. Besides the core of a respiratory CPG (pre-Botziner and Botziner complexes) a distinct source of neural oscillations, the parafacial respiratory group (pFRG) that overlaps with the retrotrapezoid nucleus (RTN), has been proposed to play a role in respiratory function. RTN/pFRG oscillations are hypothesized to evoke pre-inspiratory (or late-expiratory) discharges in the abdominal motor output during forced expiration, but the exact physiological role of these oscillations, the conditions for their emergence, the mechanisms of interactions between the BotC/pre-BotC and RTN/pFRG oscillators, and their role in generating coordinated inspiratory-expiratory activity remain poorly understood. This project is aimed to (1) refine our understanding of the spatial and dynamical organization of different neuron phenotypes and their interactions within the BotC/pre-BotC core of the respiratory CPG, and (2) define the metabolic/physiological conditions for the emergence of RTN/pFRG oscillations and their interactions with the BotC/pre-BotC respiratory oscillations. These aims will be addressed by studies employing a unique combination of different experimental methods applied in vitro and in situ, including functional and structural imaging, intracellular recording from, and selective viral vector-based pharmaco- and opto-genetic manipulation of, BotC/pre-BotC and RTN/pFRG circuits. My role in this project is to develop a computational model of this system assimilating the data received in the experiments which may serve as a computational framework and test bed for formulating predictions about abnormal respiratory behaviors. This project is currently funded by NINDS/NIH (2006-2011 R01 NS057815 Rybak (PI), Drexel U College of Medicine) and will be submitted for renewal to NIH in March 2011.
Neural oscillations with various temporal and/or spatial synchronization properties play fundamental roles in brain function, including sensory processing, central mechanisms, and motor control. Discovering the nature of existing or emerging oscillations, and analyzing the complex, state-dependent interactions between oscillations would have a broad impact on understanding the general principles of brain operation as well as the specific neural mechanisms involved in the control of various rhythmic movements and processes. This project focuses on the investigation of two neural oscillators mentioned above. The first oscillator is the respiratory CPG and the second oscillator, termed the parafacial respiratory group (pFRG), resides in the retrotrapezoid nucleus (RTN). The RTN/pFRG oscillations, emerging under certain conditions, become phase-locked with the BotC/pre-BotC oscillations and drive forced expiratory motor activity. The complexity of our computational models, based on explicit simulation of populations of neurons modeled in the Hodgkin-Huxley (HH) style, does not allow implementation of dynamical systems methods for theoretical investigation of the possible states and oscillatory regimes in the system. In this project we reduce that model so that the simplified one maintains the essential features and architecture of the large-scale model, but relies on simplified activity-based descriptions of neural populations. This simplification allows us to use methods of dynamical systems theory, such as fast-slow decomposition, bifurcation analysis, and phase plane analysis, to elucidate the mechanisms and dynamics of synchronization between the RTN/pFRG and BotC/pre-BotC oscillations. Our particular interests concern physiologically relevant behaviors reflecting specific changes in metabolic and/or physiological conditions. This study was a basis for a grant proposal of our lab in collaboration with Dr. Jonathan Rubin (University of Pittsburgh Dept. of Mathematics) submitted to CRCNS NSF program and received almost the highest score possible but was not funded because the NSF program ran out of funds. This proposal will be resubmitted as soon as the program is renewed.
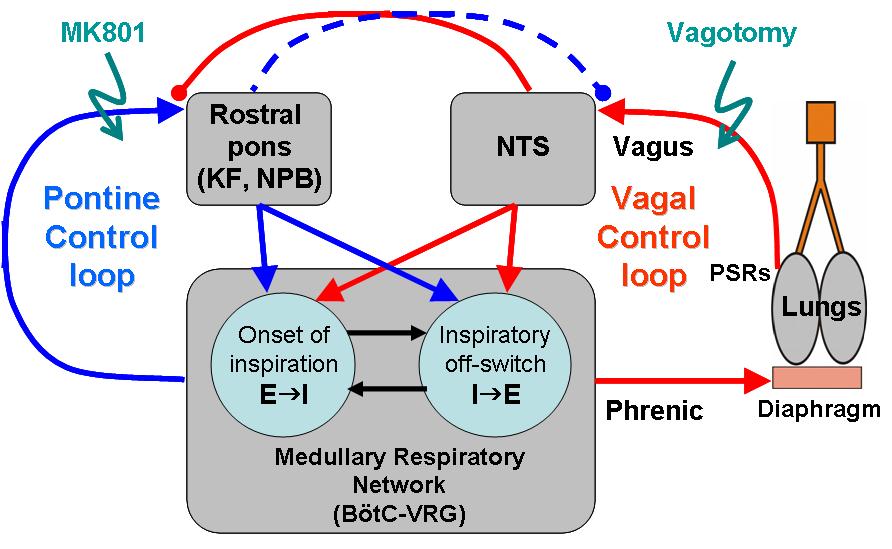
Different temporal patterns of respiratory motor activity are produced for sufficient ventilation under various conditions, including cases of hypoxic/hypercapnic stress. The long-term objective of this project is to uncover the neural mechanisms by which respiration pattern and frequency is controlled by two interacting feedback loops: the peripheral afferent loop and the central pontomedullary loop. In order to achieve this objective, our existing computational model is being significantly extended to include specific afferent receptors and their postsynaptic targets in the nucleus tractus solitarius (NTS), and this circuitry is connected to the formidable central pattern generator (CPG) circuitry currently in our model. Next, a model of pontine circuit elements is created and integrated with the CPG circuitry. Finally, synaptic connectivity between the NTS and pontine circuits is incorporated into the model. The model is used to generate specific hypotheses regarding control of phase-switching (expiration-to-inspiration and vice versa), phase duration, and motor pattern across three respiratory output nerves (the phrenic, hypoglossal, and abdominal vagus). In the experimental part of this project those hypotheses are tested. Namely, first, pontine inputs to the NTS will be blocked pharmacologically (in vivo) or via transection (in situ), in order to test the hypothesis that information regarding pulmonary distension and arterial blood gas tension carried by individual NTS neurons is modulated by the pons. In the second major series of experiments our collaborators record the responses of respiratory CPG neurons to hypoxia alone and hypoxia with pulmonary distension, before and during blockade of pontine inputs to the NTS. In the third experimental series, they record from pulmonary stretch receptor (PSR) and arterial chemoreceptor (ACR) second-order neurons in the NTS during naturalistic stimulation before and during pharmacological inactivation of the RTN/pFGR region. Then, pharmacological blockade or electrical stimulation in the NTS is supposed to be used to test the hypothesis that NTS activity alters control of respiratory frequency and pattern by pontine neurons. Using analysis of both single-unit activity in the Kolliker-Fuse and parabrachial nuclei and motor phase/frequency constancy, we are going to quantify these changes in the presence and absence of ACRs and PSRs, with and without NTS projections to the pons. In the subsequent experimental series, specific sub-nuclei in the NTS will be selectively inactivated before and during hypoxic challenges in order to dissect the role of the NTS in pontine control of the CPG by recording of single neurons in the pons. Finally, electrical stimulation will be used to identify which specific neuron classes in the CPG receive direct input from the pons or the NTS, and if a common class receives convergent input from both sites. In summary, my computer simulation and analytical efforts combined with neurophysiological and neuropharmacological experimentation of our collaborators will be used in a systems biology approach to unravel the complex neural interactions that control various aspects of respiratory rhythmogenesis and pattern formation. The proposed work has a direct impact on treatments of acute anoxia, central apnea, SIDS, and other neurogenic respiratory ailments. This project is submitted as a grant proposal to NIH in collaboration with Thomas E. Dick, Case Western Reserve University and Robert F. Rogers, Drexel University and is currently pending.
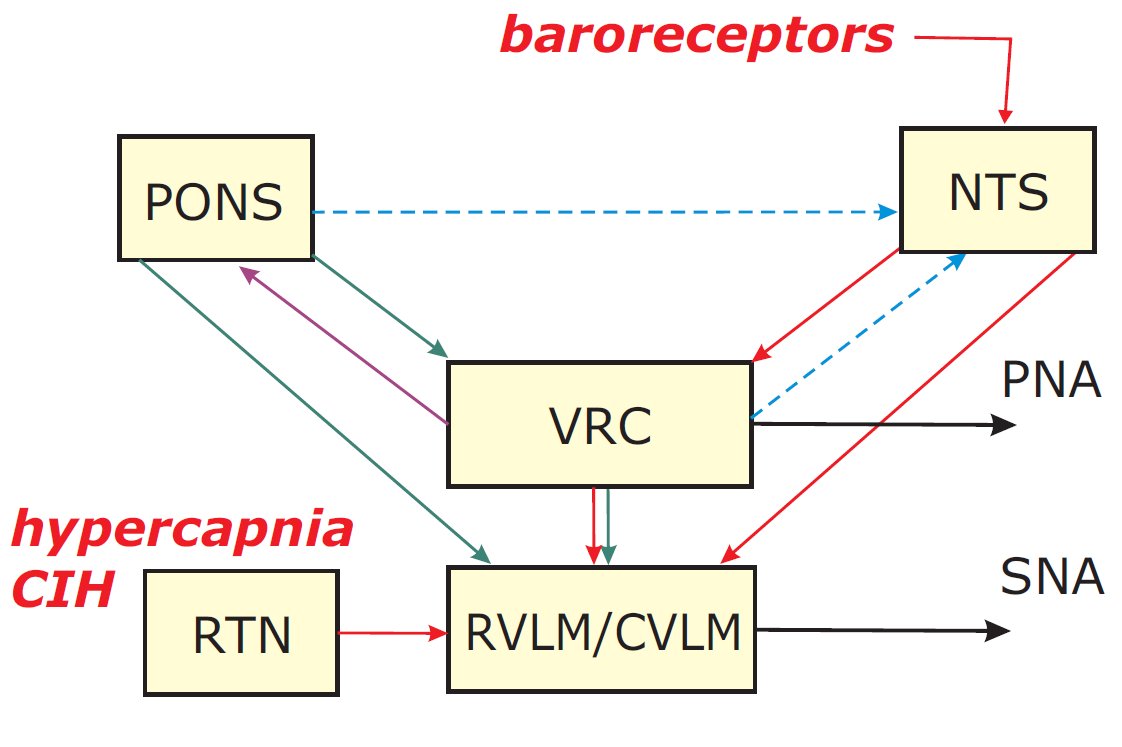
The goal of this project is to elucidate the neural mechanisms of coupling between the respiratory and sympathetic control systems, which are involved in augmenting sympathetic activity associated with hypertension. It is well-established that sympathetic nerve activity (SNA) is coupled with respiration. Interactions between respiratory and cardiovascular functions, mediated in part via respiratory-modulated changes in SNA, are essential for efficient gas exchange. The mechanism is unknown, but some forms of hypertension, including those caused by sleep-disordered breathing (SDB) elevate respiratory modulation of SNA. A CO2-sensitive chemoreceptive area in the rostral medulla, the retrotrapezoidal nucleus (RTN), co-activates SNA and abdominal motor nerve activity (AbNA) during late expiration, an activity expressed in hypertensive animals and in normal animals only during hypercapnia/hypoxia. Previously the RTN and the co-localized para-facial respiratory group (pFRG) were thought to provide tonic rather than respiratory-modulated SNA excitation. The late-expiratory oscillations in the RTN/pFRG may be suppressed by medullary circuits of the respiratory pattern generator (RPG) under normal conditions, but restored during hypercapnia/hypoxia to influence rostral ventrolateral medulla (RVLM) activity and SNA. We therefore propose to identify the neural substrate of respiratory-modulated and hypertension-associated SNA elevations, by combining ensemble recordings, multiple electrodes recording single neurons from the ventral respiratory column (VRC), RVLM and caudal ventrolateral medulla (CVLM) in the perfused in situ rat preparation with advanced computational modeling. The following questions are adressed: Is RTN/pFRG drive involved in the respiratory (late-expiratory) modulation of RVLM or CVLM neurons? Are RTN/pFRG neurons sensitized to the altered extracellular CO2 levels caused by chronic intermittent hypoxia? Finally, does the pontine dependence of respiration-SNA coupling reflect a pontine role in disfacilitating RTN/pFRG neurons and/or pontine input to RVLM neurons, and/or pontine input to the core CPG elements? My role in this project is to develop a large-scale computational model of the brainstem respiratory network that incorporates lateral pontine, RTN/pFRG, BotC and pre-BotC compartments together with RVLM and CVLM compartments and focus on simulations of their interactions, closely coordinated with experimental studies of our collaborators - Thomas E. Dick, Case Western Reserve University and Daniel B. Zoccal, Federal University of Santa Catarina, Brazil. Specifically they are supposed to confirm our model predictions that 1) neurons of the RTN/pFRG and also the Botzinger and pre-BotC complexes project to RVLM and CVLM neurons, thereby providing respiratory modulation of SNA; 2) inputs from the dorsolateral pons, including the Kolliker-Fuse (KFN) and lateral parabrachial nuclei, influence activity of RVLM and CVLM neurons directly, or via RPG neurons. The proposed collaborative multidisciplinary study should provide important insights into the neural mechanisms of respiratory-SNA coupling and may suggest improved treatments for hypertension and related cardio-respiratory disorders. This project will be submitted as a grant proposal to NIH in March 2011.
Sepsis-induced inflammation is associated with a poorly understood, sequential loss of organ function that highlights the complexity of acute illness. Acute respiratory distress syndrome (ARDS) is one component of this process, and is due, in part, to excessive or maladaptive inflammation. Such a disease as ARDS affects nearly 88,000 patients per year and claims the lives of nearly 30% of these patients. In the proposed Program Project "Sepsis/ARDS: From Pattern to Mechanism and Back" (Program PI Dr. Vodovotz, Director of Center for Inflammation and Regenerative Modeling (www.mirm.pitt.edu/cirm), University of Pittsburgh), in collaboration with multiple institutions we will model the neuro-inflammatory process by which sepsis/acute respiratory distress syndrome (ARDS) can lead to reduced heart rate variability (HRV) and breathing pattern variability (BPV), and by which reduced physiologic variability in turn affects inflammation. We will develop and experimentally validate computational models of the reciprocal interactions among the heart, lung, and nervous system in the setting of experimental sepsis/ARDS.
My responsibility is a modeling part of a subproject orchestrated by Dr. Thomas Dick, Case Western Reserve, briefly described as follows. Deterministic variability in physiologic signals is a fundamental and relevant aspect of cardiopulmonary function, and reduced HRV is predictive of mortality after sepsis. Respiratory modulation of HRV includes an increase in heart rate (HR) during inspiration or respiratory sinus arrhythmia (RSA) and a significantly increased tendency for heartbeats to occur just prior to an inspiration or cardio-ventilatory coupling (CVC). Our subproject is aimed to develop nonlinear dynamic approaches to analyze HRV and BPV. Our working hypothesis is that variability of cardio-ventilatory rhythms reflects status of cardio-pulmonary health, and that specific, predictable, disruptions in these rhythms are indicative of sepsis/ARDS. We propose 1) to define (in rats, swine, and humans) the sepsis-evoked neuro-immune response that results in a decreased BPV and decreased high-to-low ratio in HRV; 2) to determine if biologically variable ventilation alters inflammation and improves outcomes following sepsis; 3) to apply our established mathematical models of respiratory rhythm generation in order to explore the interaction between vagal processing of sensory information, generation of BPV, and the expression of HRV. We are going to establish that gain changes in vagal afferent inputs to the nucleus tractus solitarii (nTS) affect the subsequent processing of sensory input and alter HRV and BPV. The neuro-immune response is triggered by vagal-retrograde transport of cytokines to NTS as well as central production of inflammatory cytokines. My preliminary modeling findings are how HRV depends upon pontine input to the brainstem, and a further step is determine how this could act through direct and indirect pathways with preganglionic vagal neurons to alter HRV in sepsis/ARDS. Importantly, this project will also provide data how biologically variable ventilation affects the inflammatory response, thereby defining the physiological mechanisms that underlie the positive feedback loop of inflammation - decreased HRV and BPV - inflammation.
This work is currently funded by HBLI/NIH (2006-2011 R33 HL087379). The program grant will be submitted to NIH in January 2011.
The mechanisms of rhythmogenesis in the brainstem and spinal cord in vitro remain poorly understood. In this study, we extend existing models of excitatory neural populations incorporating different combinations of cellular mechanisms responsible for burst onset and termination/ recovery that may operate in the brainstem and spinal cord. The persistent sodium current (INaP) has been found in these regions and it is believed to play a role in rhythm generation. Another experimentally identified source of rhythmic activity is concerned with changes in intracellular calcium. Recently a model incorporating INaP-dependent bursting mechanism and IP3-dependent calcium oscillations was suggested by Toporikova and Butera, Georgia Institute of Technology. They demonstrated different bursting mechanisms which can arise in such a model neuron depending on the injected current and neuromodulator concentration. We are specifically interested in exploring the properties of excitatory (glutamatergic) networks of bursting neurons in brain stem because they play a critical role in rhythm generation at least under some conditions. The interactions in such a network are represented by glutamatergic synapses. On the other hand, neurons are known to have metabotropic glutamate receptors (mGluR1) responsible for IP3 production. So, using corresponding biochemical models available in literature we have built a model in which both INaP-dependent bursting and IP3-dependent calcium oscillations are controlled by incoming glutamatergic inputs. Attaching spiking activity of such a unit to itself one can mimic some properties of reciprocally excitatory network consisting of such neurons. It is of our particular interest to determine how interplay between different oscillatory mechanisms operating in the network determine changes of its bursting frequency and its duty cycle. This project is supposed to underlie my application for an NSF grant in January 2011.
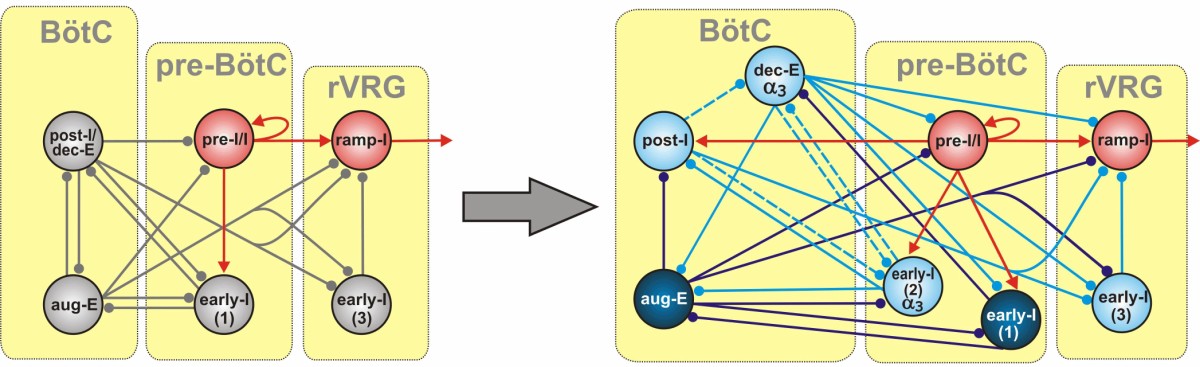
It is now widely accepted that inhibitory interactions play a critical role in respiratory rhythm generation but the data concerning the types of neurotransmission involved remains contradictory and inconsistent. The discovery of serotonine receptors 5-HT1AR which are abundantly co-expressed with specific glycine (one of the two wide-spread inhibitory neurotransmitters) receptors GlyRα3 seems to help reconstruct the connectivity of the respiratory CPG in terms of different neurotransmission types. Using our core circuitry of the respiratory CPG and distributing different types of neurotransmitters between different inhibitory neural populations we are able to explain the data obtained in the experiments when a 5-HT1AR agonist (8-OH-DPAT) or specific neurotransmitter anatogonists (e.g. strychnine) were applied. Those experiments have a very interesting "side effect", namely, 5-HT1AR agonists may effectively protect against opiate-induced apnoea or severe slowing of breathing creating the possibility for a treatment. This is another important aspect that our model must account for. This study is conducted in collaboration with Prof. Dr. Diethelm W. Richter, Univ. Goettingen, Germany and is supposed to become a grant proposal to CRCNS US-German collaboration program in February 2011.
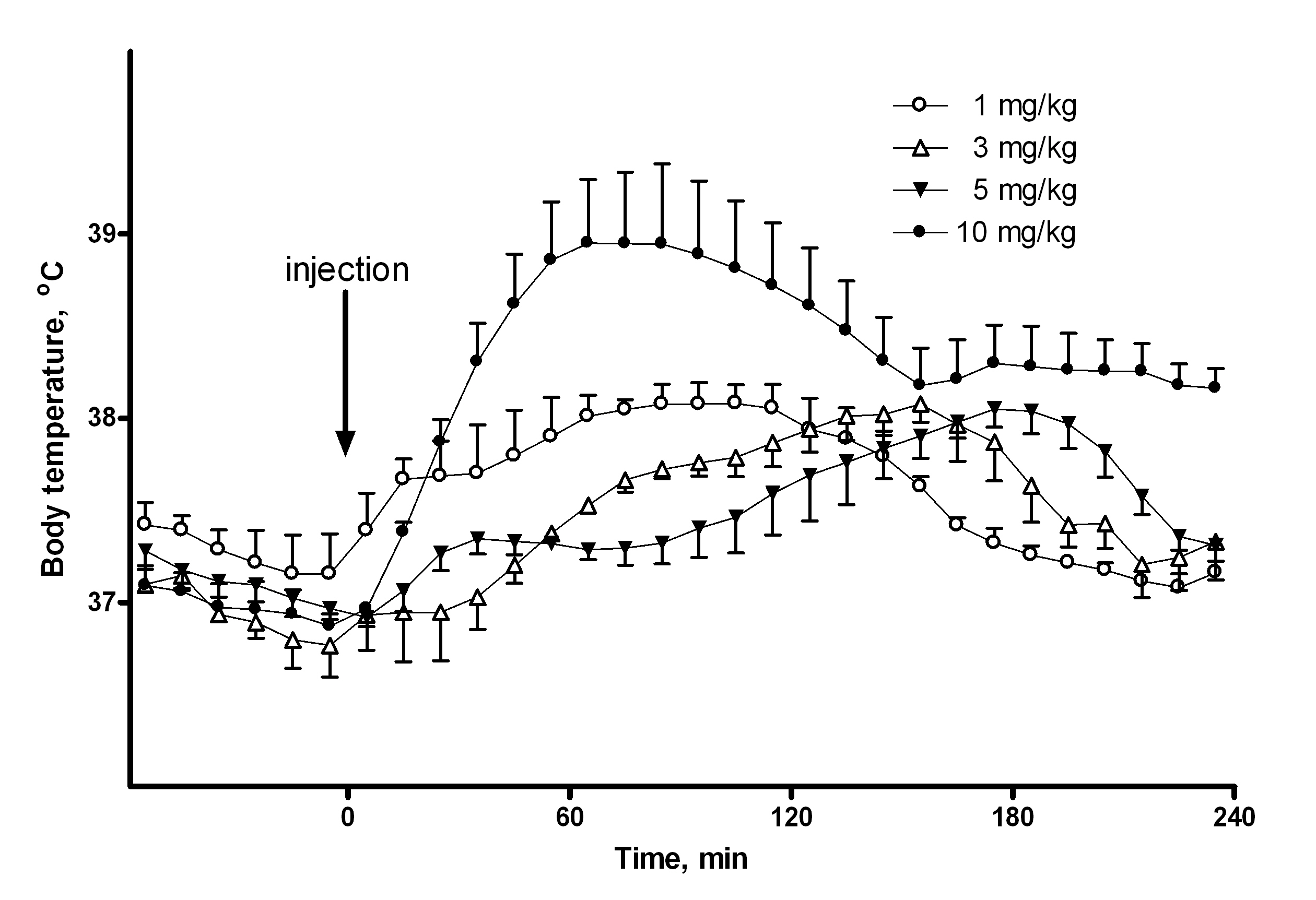
Derivatives of amphetamines are widely abused all over the world. After long-term use they lead to cognitive, neurophysiological, and neuroanatomical deficits. Neurophysiological deficits are enhanced by hyperthermia, which itself is a major mortality factor in drug abusers. Temperature responses to injections of methamphetamine are multiphasic and include both hypothermic and hyperthermic phases, which are highly dependent on ambient temperature and previous exposure to the drug. Also, various derivatives directly affect various neuromediator systems, such as dopaminergic, noradrenergic, serotonergic. Finally, body temperature is dependent on multiple thermoregulatory mechanisms and complex neuronal circuitry. Not surprising that studying effects of amphetamines is very difficult due to multiplicity of factors involved. Most of research is focused on simplified experimental settings which do not have any predictability on real-life situations. We consider modeling as a breakthrough tool to design studies of translational value. The long term goal of our project is to construct a comprehensive and physiologically relevant model of doze-dependent temperature response to methamphetamine representing interconnected neural structures which are experimentally proven to be specific brain areas and cell groups. We will start with generating a pilot set of experimental data on effect of inhibition of neuronal activity in the dorsomedial hypothalamus of the rat on temperature responses to a single dose of methamphetamine. Combining our existing pilot data and the data obtained in this project we will prepare an application for investigator-initiated grant of R01 type from National Institute of Drug Abuse, NIH.
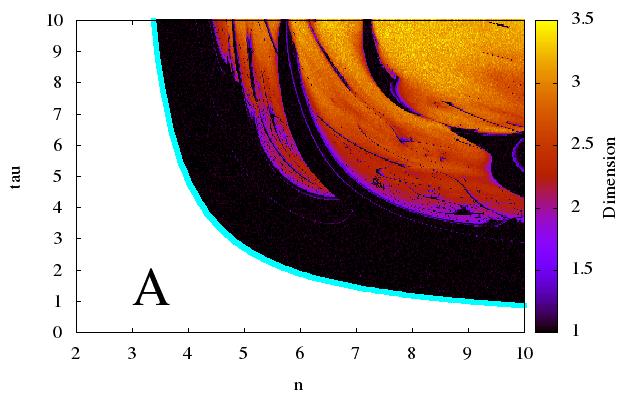
Genetic oscillatory networks can be mathematically modeled with delay differential equations (DDEs). Interpreting genetic networks with DDEs gives a more intuitive understanding from a biological standpoint, but it presents a problem mathematically, for DDEs are by construction infinitely-dimensional and thus cannot be analyzed using methods common for systems of ordinary differential equations (ODEs). In our study, we address this problem by developing a method for reducing infinitely-dimensional DDEs to three-dimensional ODEs. We find that the reducibility of a DDE corresponds to its robustness. For non-robust DDEs that exhibit behavior characteristic of high-dimensional systems of ODEs, we calculate analytic dimension lines to predict the dependence of the DDEs’ effective dimension on parameters. From these lines, we deduce that the dimension of non-robust DDEs grows linearly with the delay. On the other hand, for robust DDEs, we find that the period of oscillation grows linearly with delay. We find that DDEs with exclusively negative feedback are robust, while DDEs with feedback that changes its sign are not robust. We find that non-saturable degradation damps oscillations and narrows the range of parameter values for which oscillations exist. Finally, we deduce that natural genetic oscillators with highly-regular periods likely have monotonic promoters.
This project represents the first steps towards the ultimate goal of creating a comprehensive multi-level, multi-scale neural model of the motor control system. The model will ultimately serve as a test bed for studying motor dysfunctions associated with and/or resulted from Huntington’s disease (HD). We will take a multi-resolution modeling approach in which more detailed cellular and network neuron models (representing various CNS regions) will be sequentially incorporated into a larger systems-level model that produces simulated movements. The initial low-resolution systems-level model will describe system interactions between the major brain structures (nuclei, represented as model compartments) involved in planning, learning, and control of individual and successive arm reaching movements. We will consider (simulate and investigate) multiple interrelated motor/behavioral processes involving the basal ganglia (BG) including goal-directed reaching movements, decision-making between competing motor strategies, and motor task execution. The resultant model will provide a test bed for subsequent simulations of sequential reaching-related motor dysfunctions associated with HD, as well as for predictions of the possible effects of HD treatments at various functional levels.
My dissertation addressed problems of reconstruction of dynamical systems from time series with the main goal of prognosis of their future qualitative behavior. Such an approach does not require full and detailed information about the underlying physical processes so potentially it makes possible to build the model directly from experimental measurements. Although many researchers approached this problem during last 30 years there was no solutions successfully applicable to real “natural" data. There are three main fundamental reasons for this: noisiness of the data, too high model dimension required, and the presence of stochastic component in system dynamics. The goal of my work was to find the solutions to these problems applicable to real data. The work was carrying out under the supervision of Prof. Alexander Feigin (Institute of Applied Physics Russian Academy of Sciences (IAP RAS), Russia).
The noisiness of the experimental data requires using Bayesian approach to obtain unbiased estimates. Bayesian approach to inverse problem solution takes into account both nature of the noise and a-priori information about the system in statistically correct way. Although the original Bayesian approach is known to be not directly applicable to chaotic systems, we suggested a modification that allowed using it in the most effective way. Based on this modification, I worked out a method of reconstruction of dynamical system from weekly non-stationary chaotic time series containing essential measurement noise. The method is based on constructing time-dependent parameterized model of evolution operator in the form of neural network which is able to reproduce a non-stationary dynamics of reconstructed dynamical system. Markov Chain Monte Carlo method is utilized for the investigation of posterior Bayesian distribution of neural network parameters.
One of the applications of this method is the problem of prognosis of dynamical system behavior from time series when the character scale of non-stationarity is much greater than observation time. We have shown that probabilistic prognosis of a system behavior for times greater then observation time interval can be still informative. Some restrictions as well as possible advances of suggested method had been considered.
A common problem when reconstructing dynamical systems from noisy chaotic time series is to determine appropriate embedding dimension. The available techniques of determining embedding dimension (the false nearest neighbor method, calculation of the correlation integral, and others) are known to be inefficient, even at a low noise level. I proposed a new approach based on constructing a global model in the form of an artificial neural network. The required amount of neurons and the embedding dimension are chosen so that the description length should be minimal. The considered approach is shown to be appreciably less sensitive to the level and origin of noise, which makes it also a useful tool for determining embedding dimension when constructing stochastic models.
My most recent work in collaboration with the same team is the generalization of Bayesian reconstruction approach to the case of stochastic dynamical systems. It remarkably widens the class of the systems since the ones influenced by random perturbations are also included. Besides, as we showed, this class of systems can be useful for modeling of high-dimensional deterministic dynamical systems. We formulated a consistent Bayesian approach to modeling stochastic (random) dynamical systems by time series and implemented it by means of artificial neural networks. On model examples we demonstrated a feasibility of this approach for both, creating models adequately reproducing the observed stationary regime of system evolution and predicting changes in qualitative behavior of a weakly non-autonomous stochastic system. We also showed that some basic limitations arising in the case of deterministic systems may be reduced substantially for stochastic systems. In particular, we demonstrated a successful prognosis of the more complex system’s behavior than the observed one, which is impossible in principle for deterministic dynamical systems.
Working for the department of atmospheric research in IAP RAS in collaboration with Prof. Alexander Feigin I suggested applying Bayesian approach to retrieval of vertical ozone profile from radiometry data that allowed solving the corresponding inverse problem in statistically correct way. We developed this method based on parameterization of an unknown distribution by a neural network and using Monte Carlo Markov Chain (MCMC) technique for the analysis of a posterior Bayesian distribution. We showed that using this method one can get unbiased estimate of vertical ozone profile and its deviation as opposed to previously used methods. Currently the developed algorithms are being adapted for using with serial ozonometers.
I was also involved in investigations in the field of atmospheric photochemistry. It is known that the most of chemical models are characterized by extremely large number of equations and different time scales that makes their computational investigation very difficult. In collaboration with Prof. A.M. Feigin and Dr. I.B. Konovalov (IAP RAS) we proposed an asymptotic approach that allows significantly reducing the number of independent variables of such systems. Using that approach we built essential dynamic model of the mesospheric photochemical system and proved its dynamical properties to be identical to original ones in the wide range of control parameters.
My scientific work started by the investigation of fluxon dynamics in a long Josephson’s junction described by the perturbed sine-Gordon equation. In collaboration with A.G. Maksimov (now at SU Higher School of Economics, Russia) and V.I. Nekorkin (Institute of Applied Physics of Russian Academy of Sciences (IAP RAS), Russia) we investigated the dynamics of kink solutions to the perturbed sine-Gordon equation. Using qualitative methods of differential equation theory and based on numerical simulations, we found several types of kinks and the dependencies of their propagation velocity on the bias parameter. We found numerically that these dependencies have a scaling property, so it made its possible to estimate the lower boundary of the parameter region where the kink solutions can exist. We explained the form of the kink solutions and discussed their dynamics.
My biological education started with the investigation of synchronization in models of coupled neurons in collaboration with M.M. Sushchik and Prof. M.I. Rabinovich (University of California, San Diego, USA). The goal of this research was to answer the question: if basic properties of small neural systems are determined by the individual properties of neurons or by the coupling mechanism (and to what extent). In this framework we investigated the effects related to the influence of the couplings on the oscillatory frequency of interacting neurons in the context of general properties inherent in different models. For broad class of models we found the effects similar to those observed in the Hindmarsh-Rose model. In particular, we demonstrated that the stepwise dependence of frequency on the value of inhibitory coupling occurs both at mutual synchronization of two neurons each of which is described by the same model (Hindmarsh-Rose, Sherman-Rinzel, Chay) and at interaction of the neurons described by different models (Sherman-Rinzel and Chay models).
My other project was the investigation of the qualitative behavior of the human locomotion central pattern generator. The work was carried out in collaboration with experimentalists from the laboratory of Dr. Gurfinkel (Laboratory of Neurobiology of Motor Control, Institute for Information Transmission Problems RAS, Moscow) and my colleagues at Institute of Applied Physics (IAP) Russian Academy of Sciences Mikhail M. Sushchik, Alexander Kozlov (now at Royal Institute of Technology, Stockholm, Sweden) and Alexey Kuznetsov (now at Indiana University-Purdue University Indianapolis, USA). The result of this project was building of a dynamical model of locomotor-like movements of human evoked by muscle vibration. The experiments showed very interesting features of the CPG: two metastable states that correspond to the forward and backward locomotion were obtained along with chaotic switching between these two states for some of patients. The constructed model qualitatively explained these dynamical features.
In collaboration with my colleagues at Institute of Applied Physics Russian Academy of Sciences Mikhail Sushchik, Alexander Kozlov (now at Royal Institute of Technology, Stockholm, Sweden) and Alexey Kuznetsov (now at Indiana University-Purdue University Indianapolis, USA) we investigated the effects concerned with interaction of structures in sparsely coupled ensembles of oscillators. In particular, we analyzed numerical solutions for systems consisting of coupled van der Pol-Duffing oscillators with nonlinear coupling. We studied how drive and response systems in the form of long oscillator chains can be synchronized by a small number of interconnections between chains. We showed that oscillations of the drive system oscillators and response system oscillators can be synchronized in pairs, whereas the oscillators inside each chain remain unsynchronized.